Driving HCP Engagement
Instant distribution, direct to your markets
Collaboration and knowledge-sharing are key responsibilities for the pharmaceutical industry. Innovation in technology is playing a bigger role in reshaping the way we live, work and interact with each other. In the healthcare industry, advances in artificial intelligence as well as digital technology, data and analytics are enabling researchers to explore and interpret increasing volumes of data more efficiently. However with the digital revolution comes challenges.

Don't lose The Personal Touch
The main role of pharma commercial and medical functions is to engage with HCPs to share the latest scientific content and product information. A key part of the strategy to achieve that is by sharing the latest peer-reviewed scientific content evidencing the efficacy, safety and other facets of their product for the particular indication the HCP specialises in.
Digital communication can present barriers. You can lose the personal touch – the tailored communication and immediacy of feedback that in-person contact gives you. It is therefore important for the marketers to ensure the strategy is right for using ePrints. The remote meeting scenario has resulted in a significant demand for digital content but it needs to remain relevant and timely to add value to the HCP.
This digital content may appear easier to share, however, marketers must be aware of the licence agreement for each publisher to ensure they know if sharing of content is permitted. Routine content exchanges can expose an organisation to the risk of copyright infringement if sharing takes place without permission.
In addition, some countries have introduced a transfer of value clause, where anything a pharma rep shares with an HCP that is considered to have an intrinsic value must be both accepted by each HCP it is shared with, as well as recorded formally by the pharma company. Currently this only applies in the US and France, where the Sunshine Act and EFPIA Disclosure Code dictate that any transfer of value must be recorded and disclosed. However, similar monitoring of value transfer is being considered across other countries in Europe.
“Trustrack is agile and responsive, allowing us to quickly ramp up to meet increasing demand. To date we’ve seen a 75% increase in rep usage, with the solution now implemented across the UK, Ireland and France, along with Spain.”
Gerard Akkerhuis, Senior Vice President Partner Management and special projects at Daiichi Sankyo Europe
Introducing
Trustrack is a platform that enables you to share copyright content, allowing pharma to compliantly distribute and track peer-reviewed scientific publications to drive meaningful engagement with HCPs.
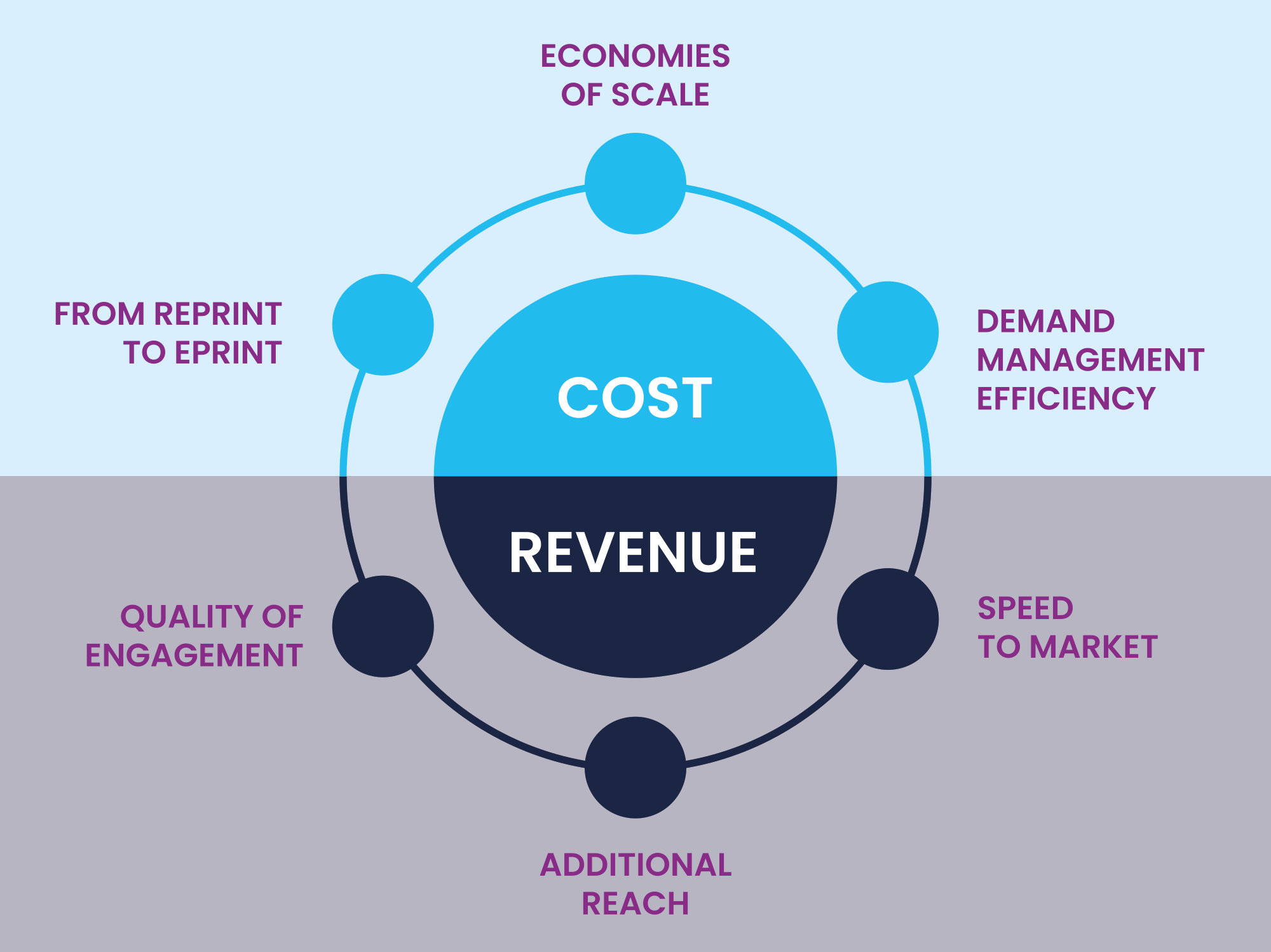
Trustrack addresses six key digital challenges relating to the distribution of ePrints:
It provides a simple, cost-effective solution, enabling reps to maintain engagement with HCPs, register every transaction in compliance with local regulations, such as the US Sunshine Act or EFPIA disclosure code, as well as allowing HCPs to provide their consent in only a few seconds.
It uses an omnichannel approach, enabling ePrint sharing in your existing channels, such as instant messaging platforms, mass email, banner advertising, at virtual, hybrid or in-person meetings and events, or self-service on websites. The ability to allocate content to numerous channels and then review and amend on a regular basis, helps to drive efficient use of your content licences. A key feature of Trustrack is its ability to track usage of content from the point of engagement. It provides rich data on HCP scientific content utilisation, channel use, value transfer and consent captured in the interaction. This data is normally immediately stored in your Customer Relationship Management platform (CRM) to achieve an enhanced view of the customer.
Related News


Why relevance and impact are so important for HCPs

The omnichannel revolution
Related Resources
Key benefits of Trustrack
- Use scientific content to extend the dialogue
- An omnichannel approach, direct to a destination of your choice
- Helps build trust, credibility & relationships
- Drives access and consent across multiple settings, such as rep-to-HCP engagement and at a congress
- Delivers customer insight
- Optimises compliance
- Increases speed of delivery
- Manages copyright and contract compliance
- Drives cost efficiencies for ePrint content
A truly Global Solution
Tangent90 is a Veeva Multichannel Content Partner and Silver Certified Veeva Technology Partner and has a long-standing relationship with Veeva to ensure full functional alignment of Trustrack with Veeva’s market-leading CRM. With a straightforward implementation process taking weeks, not months, it can be used for a single brand, a region, or globally across all brands.
Most importantly Trustrack simplifies a complex, yet often fundamental activity of the industry; to provide clinicians with the scientific evidence they want to see, in the way they want to see it.
Trustrack manages copyright content. It guarantees that when a pharma rep shares a reprint via the platform, they have the appropriate copyright stamp, transfer of value registered and any other contractual requirements in place to do so compliantly. It is a truly global solution that is set up to meet your local regulations, along with those of the publisher.